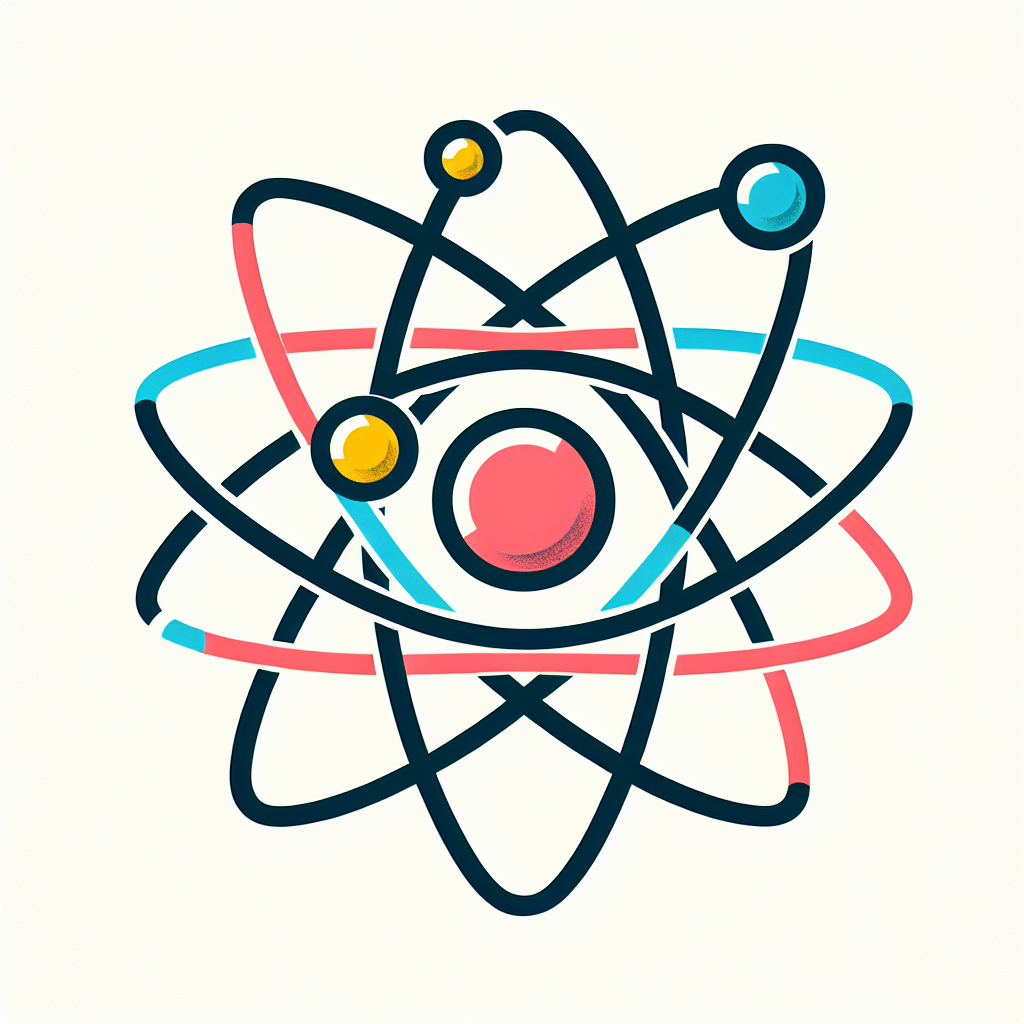
Atomic Structure
An atom is like a tiny solar system. At the center, the nucleus, akin to the sun, consists of protons and neutrons. Protons carry a positive charge, while neutrons carry no charge. Surrounding this nucleus, you have electrons, similar to planets, which carry a negative charge. These electrons orbit in different layers, or energy levels. The specific number and arrangement of these elements within an atom determine its nature, just as different planets and their positions in a solar system define its character.
Additional Insights
-

Atomic structure refers to the arrangement of particles within an atom, which is the basic unit of matter. An atom consists of a nucleus at its center, made up of protons (positively charged particles) and neutrons (neutral particles). Surrounding the nucleus are electrons (negatively charged particles) that orbit in designated energy levels. The number of protons determines the element's identity, whereas electrons influence its chemical behavior. Understanding atomic structure is crucial for grasping fundamental concepts in chemistry and physics, as it explains how elements interact and form different substances.